

SIBIONICS
Innovating for You: Revolutionizing Diabetes Care with Every Step.
Since our founding in 2015, SIBIONICS has been at the forefront of transforming diabetes management. Born from a commitment to innovation and a profound understanding of the everyday challenges faced by those with diabetes, we've consistently aimed to redefine the landscape of Continuous Glucose Monitoring (CGM) systems. By merging cutting-edge technology with designs centered around the user, we deliver solutions that not only meet needs but exceed expectations.
Today, with over 1,000,000 users across the globe, SIBIONICS stands as a beacon of reliability and innovation in diabetes care. Our global presence is a testament to the effectiveness of our CGM solutions and our dedication to making a significant, positive impact worldwide.
Looking ahead, SIBIONICS is committed to continuing our legacy of innovation. We're not just keeping pace; we're setting the pace—constantly pushing the boundaries of what's possible in CGM technology and striving for excellence with each new product we introduce.
Inovovať základné technológie, aby slúžili verejnému zdraviu
Zameriavame sa na potreby zdravotníckeho manažmentu najširšej populácie a tiež sa staráme o diagnostiku chorôb a liečebné režimy populácií so zriedkavými chorobami. Integrovali sme vynikajúci vedecký tím, aby sme neustále transformovali špičkové vedecké výskumy na medicínske produkty a slúžili verejnému zdraviu.

Technologické pohľady do neznáma prebúdzajú potenciál pre život
Na základe potrieb pacientov predstavujeme biochémiu, dizajn čipov, umelú inteligenciu, automatizáciu a ďalšie technológie, aby sme získali prehľad o neznámych oblastiach zdravotných údajov a pomohli ľuďom urobiť zmeny smerom k lepšej kvalite života.
Certifikáty
Sme odhodlaní pomáhať klientom zvyšovať kvalitu produktov a riešiť výzvy.

Míľniky a produktový rad

2023
The GS1 CGM system received the CE mark for entering the European market.

2021
The continuous glucose monitoring system (CGM) was submitted to National Medical Products Administration for registration and was approved. The practicing license of Yinchuan Sibionics Internet Hospital was approved.

2020
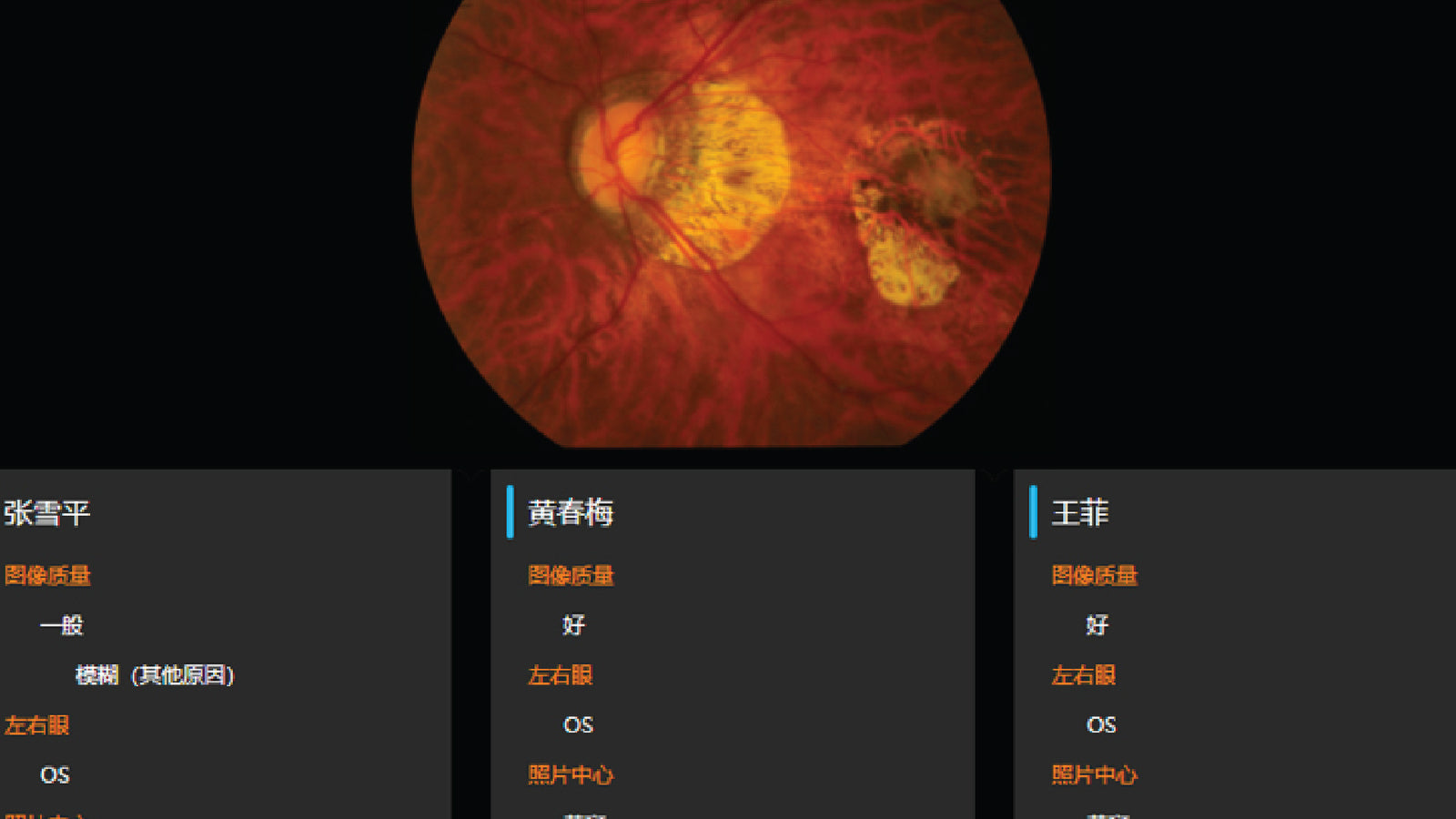
AI-DR was approved as the first Class III medical device for AI-assisted fundus disease diagnosis software in China. Sibionics was certified as National High-tech Enterprise.

2019
Fundus camera obtained the registration certificate for Class II medical device. R&D of capsule endoscopy project was started.
2016
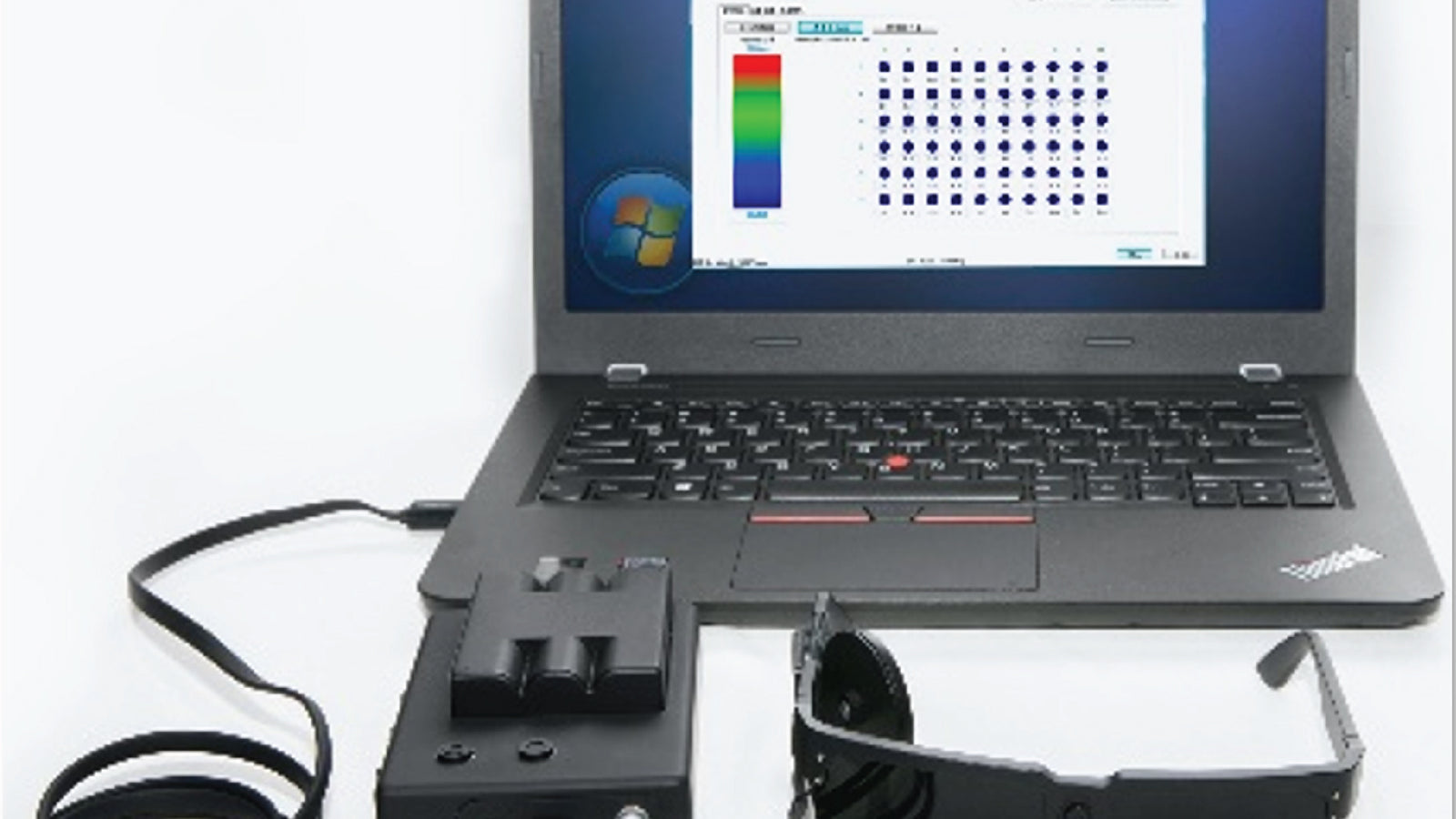
R&D of continuous glucose monitoring system (CGM) was started. R&D of diabetic retinopathy AI-assisted diagnosis software (AI-DR) was started.
2015
Sibionics was established, and American R&D Center was established. R&D of artificial retina project was started.

